Connecticut's Psychedelic Treatment Program; US Senators Press NIH/FDA for Psychedelic Plans; Phase II results of LSD for Anxiety
This week reinvigorated my conviction that this whole psychedelic thing is the most fascinating and stimulating domain to pay attention to and be involved with.
When we talk about “psychedelics” as an industry, a social movement, a field of study, an area of interest—whatever you want to call it—it is unique in that it is the intersection of many domains, each distinct, complex, and engaging in their own right:
neuroscience
healthcare systems
politics
local, state, federal, and global drug policy
the treatment of mental illness
altered states of consciousness
spirituality and religion
shamanism and indigenous wisdom
the philosophy of science
psychiatric and psychological nosology1
The list goes on, and there is no consensus, and the path forward is anybody’s guess.
This diversity of inputs and the uncertainty of future outcomes make it fascinating. It is not merely waiting for the state-by-state dominoes to eventually fall as with cannabis, and it is not simply taking a drug through the FDA approval process—there is so much more to consider with this class of substance2.
Two developments, among the many this week, were particularly captivating in this regard:
Connecticut’s Governor signed into law a bill that will establish a state-sponsored “Psychedelic Treatment Program” leveraging Compassionate Use frameworks to develop Real World Research programs and pave the way for broader post-approval adoption of PAT
and
US Senators Cory Booker (D-NJ) and Brian Schatz (D-HI) issued a letter to the FDA and NIH calling for the agencies to update the senate on federal plans for psychedelic research and funding: “We encourage NIH and FDA to further expand their role in identifying research gaps, potentially promising therapeutic uses of psychedelics, and regulatory hurdles in the field of psychedelic research.”
In this dispatch, I try to articulate why I think these are particularly compelling developments.
Beyond these, there were several other events you’ll want to be aware of:
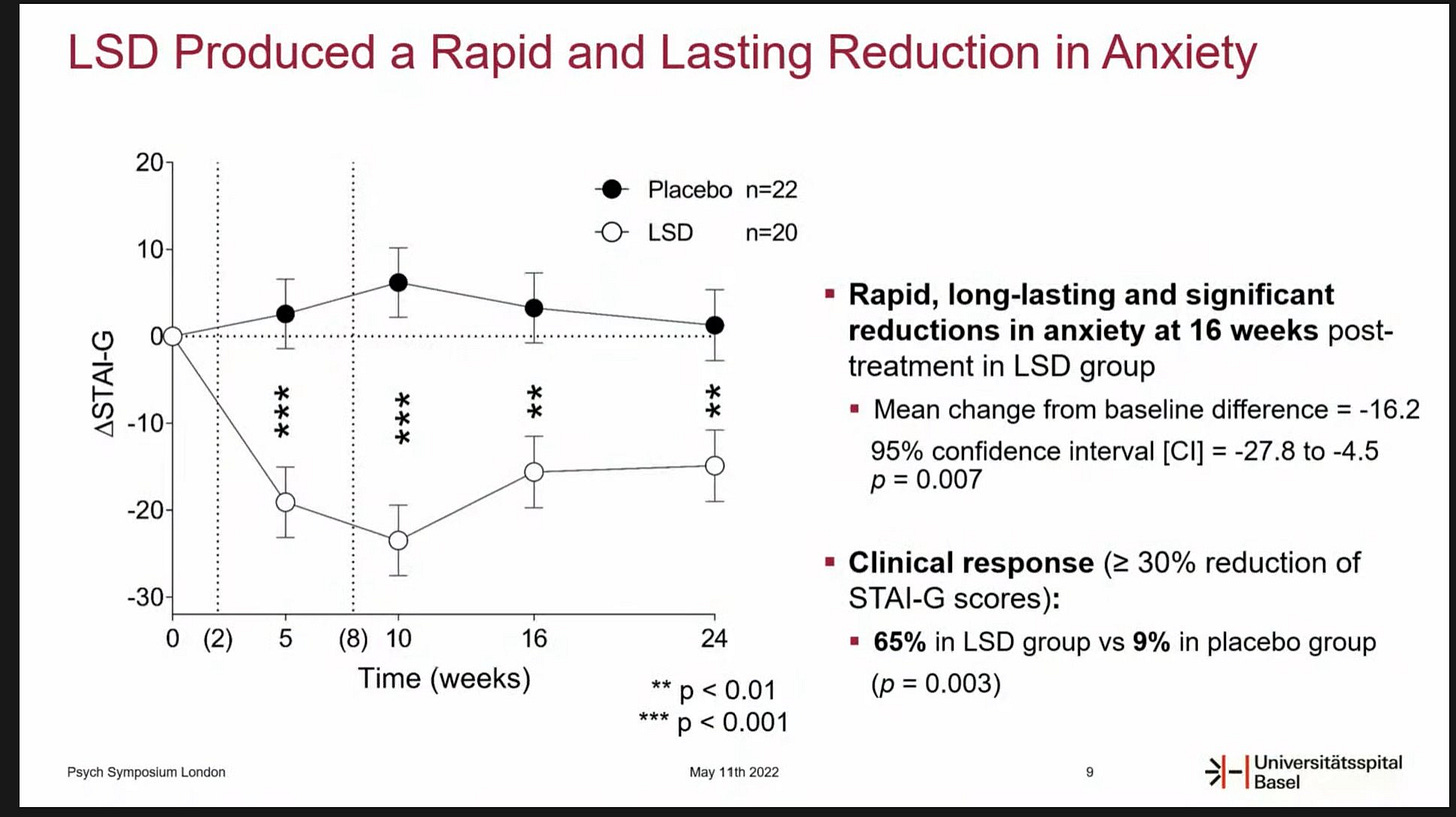
First, MindMed collaborators Matthias Liechti and Dr. Friederike Holze revealed positive topline results from their phase II study of LSD for the treatment of Anxiety: “data demonstrate the significant, rapid, durable, and beneficial effects of LSD and potential to safely mitigate symptoms of anxiety and depression.”
Interim Analysis of MAPS Phase III trial of MDMA Assisted Therapy for PTSD indicates no additional subjects will need to be enrolled and point to a 90% likelihood that final results will be statistically significant.
Peaceful protesters were arrested in front of DEA offices in Washington DC, advocating for the DEA to allow psilocybin in Right-to-Try measures.
Let’s jump in.
Connecticut’s Psychedelic Treatment Program
The governor of Connecticut signed into law a bill that will appeal to many other states considering state-level psychedelic policy reform.
Under this framework, funds from the state budget will go towards creating a Psychedelic Treatment Program through special research programs called Expanded Access—also known as Compassionate Use.
From Marijuana Moment:
“Under the bill, psychedelic treatment centers will be established in the state where people can receive psilocybin-assisted or MDMA-assisted therapy as part of an expanded access program for investigational new drugs through the federal Food and Drug Administration (FDA).
While the legislation won’t legalize the psychedelics, it will set up a regulatory infrastructure to enable Connecticut to play a leading role in providing access to this alternative treatment option as federal agencies continue to fund and facilitate clinical trials.
Psychedelic therapy will be specifically provided and funded for military veterans, retired first responders, and health care workers under the budget measure.”
When I first read about the framework, it immediately struck me as a sensible and practical way of increasing the research footprint through Real-World Research since it leans on pathways that are already federally legal, specifically Expanded Access:
"Sometimes called “compassionate use”, expanded access is a potential pathway for a patient with an immediately life-threatening condition or serious disease or condition to gain access to an investigational medical product (drug, biologic, or medical device) for treatment outside of clinical trials when no comparable or satisfactory alternative therapy options are available."
Unlike other decriminalization deprioritization and legalization measures, the Connecticut framework, while limited in the size and scope, will operate within the confines of current Federal laws—an important feature because, as we noted last week, while there may be the appearance of changing attitudes on drug policy at state and local levels, the federal government may be digging in their heels for a fight.
And because Expanded Access Programs are technically research programs, they need to be authorized by the FDA.
However, in the case of MAPS, the only organization that has established an Expanded Access Program3 that I am aware of, the FDA has capped the enrollment at 50 patients.
So the Connecticut framework, and others that follow in its path, would actually become partners with organizations such as MAPS to work with the FDA to increase enrollment caps.
State Policy & Commercialization
And this is where this project gets interesting—the potential to work with organizations to create the rails for future commercialization.
This level of detail is beyond my understanding, but I’ll take a stab at why this could be the case.
‘Normal’ drug development concludes with FDA approval, and sponsors then move into commercialization.
Critical features of commercialization include provider education (marketing) and reimbursement (insurance coverage).
But there are several considerations for psychedelic-assisted therapies, including:
Status as scheduled substances
Limitations of Risk Evaluation and Mitigation Strategies (REMS)
To-be determined aspects of the Drug-Labeling
Clinical infrastructure, therapist and provider training, and certifications
and the long duration of treatments
All of which would seem to make the early phases of commercialization extremely challenging.
Therefore, if states, which stand to benefit from improvements in mental health treatment, take on the cost and burden of establishing clinical and administrative infrastructure, they could help streamline the process.
The Connecticut framework provides funding for qualifying patients while providers, administrators, and health systems can absorb the unique features of providing PAT all while collecting Real-World Data.
From the Bill:
“Approved treatment sites shall collect and submit data to the Department of Mental Health and Addiction Services, including, but not limited to, its protocols for the provision of MDMA-assisted and psilocybin-assisted treatment, training on the facilitation of such treatment, implementation of facility standards, strategies for patient protection and mitigation of drug diversion.”
An oversite board established by the Bill would:
“advise the Department of Mental Health and Addiction Services on the design and development of the regulations and infrastructure necessary to safely allow for therapeutic access to psychedelic-assisted therapy upon the legalization of MDMA, psilocybin and any other psychedelic compounds.”
Again, this is peering through the ambiguity of yet-to-be enacted policies. Still, there seems enough to suggest that the Connecticut approach could be a valuable model for other states to adopt to prepare for commercialization.
East Coast vs. West Coast Psychedelic Policy
Finally, the Connecticut approach is a practical counterbalance to the pioneering legalization paths underway on the West Coast, with Oregon's Measure 109, California's Bill 519, and Colorado's Natural Medicine Health Act.
We might even say two distinct approaches to state-level psychedelic policy reform are emerging along the stereotypical East Coast—West Coast dichotomy that has characterized the differences between cultural, business, and power centers of the respective coasts.
Tradition, legacy institutions, and prestige codify the East Coast ethos, while West Coast boasts innovation, experimentation, and novelty.
This schism is representative of the two (general) approaches to state-level policy reforms, with the Western states aiming at progressive legalization efforts. In contrast, the more reserved East Coast approaches seek to work within the previously established frameworks erected by the federal institutions.
For example, lawmakers in Maine tried to push through an exact replication of Oregon’s Measure 109 only to have the governor shoot it down earlier this year, apparently in response to concerns from the local medical community. However, a second attempt that mimics Connecticut’s approach may fit better. We’ll see.
While the more conservative East Coast approach could streamline the adoption within the healthcare systems, it is the West Coast approach that could ultimately lead to radical changes in drug policy; therefore, both are valuable.
Senators Implore NIH/FDA for an Update on Psychedelic Plans
US Democratic Senators Cory Booker and Brian Schatz sought official clarification from the NIH and FDA about how the agencies’ plans to engage with psychedelic research.
“We are writing to request information on the latest efforts undertaken by the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) to advance research on psychoactive drugs, and in particular psychedelics, and their potential therapeutic effect
We encourage NIH and FDA to further expand their role in identifying research gaps, potentially promising therapeutic uses of psychedelics, and regulatory hurdles in the field of psychedelic research.”
A primary thesis of this newsletter is that legal psychedelics will emerge in radically different formats than either Cannabis or conventional pharmaceutical drugs, the two most common comparators.
Usually, federal research is the starting point of scientific findings that are eventually developed and commercialized by private companies.
With psychedelics, federal funding has been all but absent until recently. Instead, the evidence base to date has been built with philanthropic funding, and commercial projects have only recently gotten started.
What would the injection of significant federal funding mean for the current equilibrium?
For example, if programs like Connecticut and Oregon are successful, could federal funding be put towards Real-World Research programs in addition to the more traditional basic research and investigator lead clinical trials?
Topline Results of LSD Phase II for Anxiety
This is a case in which we are receiving the topline results of another high-profile Phase II trial without the full de. Still, like the Compass results last Fall, we have a glimpse at promising and statistically significant primary outcomes.
From the Press Release:
“LSD (200 µg) treatment resulted in significant and strong reductions of STAI-G (State-Trait Anxiety Inventory global score scores) 16 weeks after treatment in the between-subjects analysis (least square mean (± SE) change from baseline difference = -16.2 (5.8), 95% CI=-27.8 to -4.5, p=0.007).”
The results were presented at the PSYCH Symposium this week, and our friends at Blossom and Psychedelic Alpha were present and captured the details, including:
Study Design: 46 patients in a randomized, double-blinded, cross over design4
Primary endpoint: State-Trait Anxiety Inventory (STAI) 16 weeks after treatment
An acute positive or Mystical Experience was predictive of therapeutic effect
One Serious Adverse Event or “acute transient anxiety and delusions”) was reported in the LSD group
A key feature of this trial was that therapy was NOT administered during the trip. Participants did work one-on-one with therapists on non-dosing days, but it seems to have been an open-ended engagement without standardization.
A MindMed spokesperson told me:
“There was no psychotherapy during the psychedelic sessions – this is not unusual. But this study included psychotherapy in both arms. And patients were allowed to continue their anxiolytics and antidepressants during the study if they were on it/them at enrollment. They could not change them, however, during the study.”
This points to the “Psychedelic Procedures” thesis, which is where I think a lot of medical psychedelic approaches will ultimately end up:
“I had begun to think the clinical workflow of psychedelic experiences within healthcare settings would increasingly look like surgical workflows (standardized, long duration, altered consciousness, recovery period, etc.) while psychotherapy, which has been a central feature of the research protocols that have gotten us to this point, will become an add-on option if the patient can afford it or insurance will reimburse it...
From an operational perspective, procedures are favorable since they have minimal marginal costs and negligible variation from patient to patient. While psychotherapy with the need for a therapeutic alliance, trust, and human connection is not exactly scalable and reproducible and definitely not objective, and most importantly, requires skilled, trained, and empathetic human therapists.”
Further Developments
Osmind Secures $40 Million Series B:
“The Series B investment was led by DFJ Growth. New investors Susa Ventures, Lachy Groom, Brent Saunders (former CEO and chairman of Allergan and current Osmind board member), Helena Goodman and Ariel Katz (CEO of H1), and existing investors General Catalyst, Future Ventures, Tiger Global and Pear VC also contributed.”
Compass to Study Comp360 for Autism
“Compass Pathways…today announced it will be funding an investigator-initiated study that will use COMP360 psilocybin to explore how psilocybin affects specific brain pathways in autistic adults. This will be the first ever mechanistic study of psilocybin in autistic adults.”
Clerkenwell Launches Psychedelic Medicine Specific Clinical Trials Site
“With the conducive regulatory environment brought about by post-Brexit sovereignty, the UK is fast becoming a central hub for psychedelic research.”
That’s it for this week’s round-up—thanks for reading!
Zach
The branch of medical science dealing with the classification of diseases
Key themes of this newsletter seek to understand the forces at play. A primary thesis is that psychedelics—as therapeutic tools, as a growing object of fascination of the culture, as tools to study the mind and brain—are coming online at an inflection point in the utility and adoption of technology in healthcare, the personalization of medicine and growing suspicion of current drug policy—all of which make for fascinating dynamics.
In a previous post, I indicated that MAPS, Compass, and Usona had established Expanded Access Programs. However, MAPS is the only one registered with ClinicalTrials.Gov.
Cross-over design means that each participant received both LSD and Placebo.