July Psychedelics & Tech News Round-Up
Welcome to the Trip Report's Psychedelic x Technology News Round-Up for July 2021.
This series aims to keep readers apprised of developments at the intersection of psychedelics and technology and offer commentary and context for how to think about this developing niche within the broader psychedelic movement.
Today we’ll peak at:
Osmind closes a $15 million series A
Cybin partners with TMS clinic & sponsors ketamine trial with Kernel
MindMed adds Biogen Digital Medicine expert to SAB
Mydelica to Launch Public Advisory Board
Just as there are many different psychedelics with different qualities, time courses, and uses, so is there a plethora of technology categories used to:
Conduct research
Monitor treatment and manage patient care
Quantify symptoms, the severity of illness, and measure improvement
Enhance effects and/or make more precise
And just as there are many practical, ethical, and nuanced considerations for the use of psychedelics, the same applies to technology.
Furthermore, and this is the premise of The Trip Report, psychedelics are coming of age at an incredibly auspicious time when the practical uses of technology have reached an inflection point in medicine, healthcare, and personal transformation, as evidenced by the adoption of digital therapeutics, virtual reality, and neurotech.
Psychedelic and psychoplastogenic therapies in combination with digital health technology, neurotech, and immersive technologies portend a promising solution set for the most difficult to treat conditions and will be increasingly used tools for personal development and personal transformation at a time when mental illness, loneliness, and despair appear to be at epidemic levels.
Furthermore, the clinical research landscape is also evolving under evolutionary pressure from novel technology. Such innovation could be used to enable drug development, support drug reform, and aid pragmatic trials.
July Psychedelics x Tech News Round-Up
18 months ago, a flurry of entrants into the budding psychedelic industry trumpeted Vertical Integration in an attempt to recreate the cannabis playbook in psychedelics, in which production, cultivation, processing, and distribution would all be done under one roof.
Since then, investors have overwhelmingly allocated capital, not to cannabis 2.0 strategies but pharmaceutical development.
Set & Setting
Not only do the fundamental qualities of classical psychedelics lend themselves to different unit economics than cannabis, but there has also been the recognition that legal1 psychedelics demand consideration for the set and setting from virtually all stakeholders.
The Oregon Psilocybin Services Initiative, the steady progress of Psychedelic Assisted Therapy in clinical trials by MAPS, Compass, Usona, and Field Trip's clinical infrastructure model advancing to the NASDAQ all point to the importance of:
Monitoring/Guiding/Therapy
Clinical infrastructure
Process and outcome data collection2
I think this framework is useful in evaluating the tech-related news coming out of psychedelia this month.
Osmind Closes $15 Million Series A
Themes: Neuropsychiatry, Real World Data/Evidence, Precision Psychiatry
In the psychedelic arena, we have at least two Electronic Health/Medical Records providers in Maya Health and Osmind seeking to serve clinicians and therapists using psychedelic medicine. Both recognize the changing landscape and the growing opportunity to make mental health care more measurement based.
At the end of June, Osmind announced a $15 million series A:
Osmind, a healthcare technology company building the first digital infrastructure for neuropsychiatry, closed $15 million in Series A funding to further develop and expand its electronic health record (EHR) and research platform. (Emphasis added)
I'll come back to why I emphasized the term "neuropsychiatric" below.
The announcement went on to note:
It will also continue to develop its research platform, which enables the discovery, development, and delivery of new diagnostics and therapeutics for treatment-resistant mental health conditions…
The software also aggregates de-identified real-world data to accelerate research and development of new mental health treatments and diagnostics such as psychedelic medicines, neuromodulation, digital therapeutics, digital phenotyping, and neuroimaging.
This points to an idea that is top-of-mind in the changing regulatory landscape: Real-World Evidence (RWE) and Real-World Data (RWD).
There is a change afoot about how regulatory bodies, like the FDA evaluate new treatments and how healthcare providers hone in on best practices.
Real-World Evidence/Data seems to mean a few things depending on the context, but in the case of Osmind, Maya, and other EHRs that are capturing patient care data from medical records, wearables, and in-situ surveys, such information can:
Inform therapists/clinicians about the best practices with psychedelics
Inform researchers about trial design and use of novel outcome measures
Help drug developers improve trial design and lower costs by using a “virtual control arm” via RWD
For example, ketamine is an approved drug finding increasing acceptance in off-label use for depression. However, it hasn’t gone through the FDA gauntlet that typically establishes safety and efficacy. This is where RWD could help. Data generated and interpreted from real-world use can inform researchers, clinicians, payers, and regulators on best practices.
Other psychedelic projects seeking to capitalize on the advent of RWD include Albert Labs, which plan to take advantage of progressive guidelines from the UK’s Medicines and Healthcare Products Regulatory Agency (MHPRA), which appears to be welcoming novel trial designs that employ Real-World Data.
Another is a proposed framework for Pragmatic Clinical Trials put forward by Robin Carhart-Harris, Adam Gazzaley, and others to leverage progressive changes such as in Oregon, to capture RWD on psychedelic use and outcomes (for more info, we covered this in The Middle Way: Tech-Enabled Psychedelic Pragmatic Clinical Trials).
Neuropsychiatry
In the fullness of time, we'll see an increasing anatomical approach to the treatment of mental health where future imaging technology, BCI, and more precise neurostimulation are used to identify and treat the "lesion" responsible for one's condition.
This is the context in which the use of the term Neuropsychiatry is important.
I’ve never heard of it, yet I immediately intuited the implication, namely making the brain the organ of the mind and the target tissue in treating mental health disorders.
"Neurology and psychiatry have, for much of the past century, been separated by an artificial wall created by the divergence of their philosophical approaches and research and treatment methods. Scientific advances in recent decades have made it clear that this separation is arbitrary and counterproductive. Neurologic and psychiatric research are moving closer together in the tools they use, the questions they ask, and the theoretical frameworks they employ. The interests of neurology and psychiatry converge within the framework of modern neuroscience."
—The Integration of Neurology, Psychiatry, and Neuroscience in the 21st Century
This push to establish a new domain is predicated on progress in attributing at least part of the causality of mental illness to specific neural networks3.
One might evaluate this more ‘anatomical’ approach to mental health represents a further deviation away from a patient-centered, Biopsychosocial model and further biomedicalization of psychiatry.
This is hopefully balanced by the sheer power of the psychedelic experience to reify the importance of psychosocial factors. That is, as more and more people undertake psychedelic journeys, especially clinicians, researchers, regulators, and the people close to them, the importance of preparation, integration, and connection is made apparent.
Cybin to Collaborate with Greenbrook TMS; Sponsors Trial w/ Kernel BCI
Themes: Neuromodulation, Brain-Computer Interfaces (BCI),
Early in July, Cybin announced a partnership with a clinical operator that specializes in the use of Transcranial Magnetic Stimulation (TMS):
Cybin today announced that it has entered into an exclusive research and development collaboration agreement with TMS NeuroHealth Centers Inc., a wholly-owned subsidiary of Greenbrook TMS Inc. Greenbrook operates 129 outpatient mental health service centers in the United States.
What is TMS?
TMS is a form of neurostimulation that excites neural networks whose apparent malaise contributes to mental illness. It has been around at least since 1984 and has been FDA-approved since 2008.
Greenbrook is a publicly traded company with over 100 TMS clinics throughout North America.
Like psychedelics, TMS and other forms of neurostimulation are predicated on the principle of neuroplasticity. However, while psychedelics appear to soften the rigidity of neural pathways, TMS applies an electromagnetic “load” to neural tissue to drive an adaptive response that hopefully leads to improved outcomes.
To my mind, psychedelics and neurostimulation represented the Yin and Yang of Applied Neuroplasticity4.
In current trials, psychedelics are paired with therapy to drive behavioral/emotional/cognitive changes, and most agree that the therapy component does ‘the work’ while psychedelics enable it to ‘take root.’
On the other hand, forms of neurostimulation like TMS, Transcranial Direct Current Stimulation (tDCS), and peripheral nerve stimulators are effective to the degree to which they modulate functional neural connections.
This highlights the trend towards ‘neuropsychiatry’ we touched on above—TMS stimulates precise brain regions according to the region's role in mental illness. For example, in cases of depression, that region is the Dorsolateral Prefrontal Cortex (DLPFC).
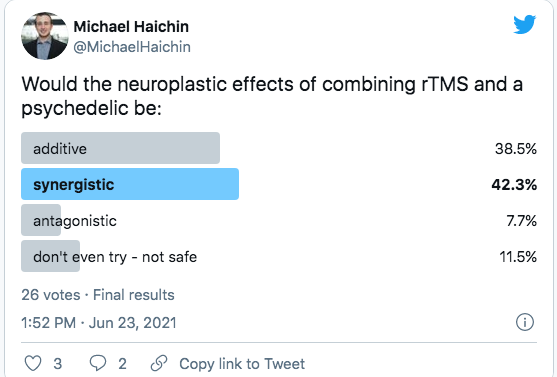
What happens when you combine the two?
Cybin appears to be asking the question, “If psychedelics make neural networks more malleable, and TMS creates targetted neuroplastic adaptations, can the two be combined to improve outcomes?”
This premise is still a premise. It has not been tested to any degree that I am aware of. Still, it is the basis of the work we expect to see from institutions like Neuroscape and DIY communities like r/RiftintotheMind that will combine psychedelics with neurostimulation or immersive experiences like VR.
Cybin x Kernel
Last year, Cybin announced a partnership with Kernel, a BCI company attempting to enable fMRI quality brain imaging through a helmet with sophisticated sensors to measure brain activity through time-domain near-infrared spectroscopy (TD-NIRS).
Basically, Kernel is attempting to make the fMRI, a large room-sized device, the size of a bike helmet and thus make brain function analysis possible in more contexts, such as during the administration of ketamine.
Recently, Kernel CEO Brian Johnson posted the following on LinkedIn:
The important theme here is the change in how conditions of the mind are defined, quantified, and measured.
In a recent dispatch, we looked at how future trials could use digital technology to measure outcomes alongside self-report surveys.
A central theme here is what might be called ‘Continuous Ecological Measurement,’ namely, the ongoing capture and distillation of everyday activities and behaviors through wearables and smartphones as proxies for mental/behavioral health. This approach goes by “Digital Phenotyping” or “Mobile Sensing” and is the basis of a handful of recent startups, including Mindstrong, Ksana, and Aware Healthcare.
The other approach to making subjective suffering more objective is to make brain activity more ‘readable.’ If researchers could use an fMRI in more situations, we’d probably know more about these conditions. If we knew more about the functional/anatomical aspects, we could target them more precisely.
This is might be called ‘the neuropsychiatric flywheel’.
MindMed Adds Biogen Digital Medicine Expert to SAB
Themes: ‘quantifying the subjective,’ decentralized clinical trials, Big Pharma’s interest in psychedelics
From the press release:
“MindMed, has announced the addition of Dr. Peter Bergethon, a world-leading expert in neurology, digital medicine, and central nervous system (CNS) drug development to the Company’s Scientific Advisory Board…
Bergethon is the Vice President and Head of Digital and Quantitative Medicine at Biogen Inc., where he leads the effort to transform clinical trials and humanize drug discovery by encouraging the transition of clinical trial measures from a qualitative to a quantitative discipline…
Quantifying the Subjective & Decentralized Clinical Trials
All of the NASDAQ/NYSE traded psychedelic developers have a technology division/products hopeful of fulfilling several purposes, including scaling access, enhancing therapeutic effect, and enabling quantification of disease state and its hopeful resolution.
MindMed’s acquisition of HealthMode in February and this appointment of Dr. Bergethon signals the importance the company is putting on innovation in technology-aided clinical trial design.
Many believe that if clinical trials adopt continuous, passive data and smartphone-delivered surveys and regulators agree to accept these new outcomes measures, clinical research would be more cost-effective, inclusive, and realistic.
The decentralization of clinical trials—which I would argue is particularly important in the psychedelic space because one day, we could see data generated from personal use be used to support drug reform and drug development—is a trend to pay attention to.
Do Psychedelics Appeal to Big Pharma?
The end of phase II is a common point for small, pre-revenue biotech startups to pass the baton to larger pharmaceutical companies with experience in Phase III, marketing, and distribution.
However, it is unclear if this trend will apply to psychedelics given their unique attributes (set & setting, Schedule I, time duration, adjunctive to therapy, etc.)
A comparable case study might be Prescription Digital Therapeutics (PDT) in that they represent a paradigm shift that pharmaceutical companies, regulators, and payers have had to adjust to.
In this context, the post-Phase II-acquisition trend appears less straightforward when the assets are digital health products5.
Consider the two most promising/interesting Digital Health companies— Akili Interactive and Pear Therapeutics.
Neither has been acquired, yet both have FDA-cleared products, and patients are using them.
Akili just raised a $110 million Series D round and $50 million in loans a year after debuting EndeavoRX, a closed-loop video game for treating ADHD in children.
Pear Therapeutics became a public company via a SPAC 4 years after the FDA approved their first DTx products.
Part of pharma’s standoffishness has to do with reimbursement—from Healthaffairs.org
“While the Centers for Medicare and Medicaid Services (CMS) policy on telemedicine has been a bellwether of change for commercial payers, the agency has yet to develop guidance for DTx reimbursement…
the absence of an established, repeatable, and scalable path to commercialization and prescription of DTx has bottlenecked broader uptake. Consequently, current DTx evaluation decisions among commercial payers are largely ad-hoc and based on individual priorities.”
Now replace DTx with psychedelics…
MyDelica to Launch ‘Friends Advisory Board’
Of the handful of mobile apps available to support psychedelic experiences and enable trippers to contribute to science, MyDelica stands out simply for the endorsement and involvement of Robin Carhart-Harris.
Mydelica recently announced a “Friends Advisory Board” through which one can be granted early access to the app and provide feedback, suggestions, and ideas.
I sense that apps like Mydelica are broadly welcomed as long as the first principle of “Do no harm” is adhered to. In this context, Do no Harm means ensuring anonymity, privacy protection, and individual data ownership.
Apps like MyDelica and Quantified Citizen seem to have interest from both consumers and researchers. However, I imagine there is tension between creating value for research and/or commercialization purposes and ensuring privacy and data ownership.
This will no doubt be a topic I’ll be going deeper on in the future.
That’s it for this dispatch; thanks for reading, sharing, and engaging!
Zach
In this context, I am using “legalization” in broad strokes to imply medicalization, efforts like Oregon’s Psilocybin Services initiative, and any other future schemas (Australia???) that would enable federal or local sanctioned sales of psychedelic substances.
I have a lot of work ahead of me to understand the role, applicability, value of personal health data captured and analyzed before, during, and after psychedelic experiences - that is, who ultimately owns it, how does enable innovation in treatment design, and contribute to the overall valuation of a company, heck these may not even be the right ways of framing the question. This will be a continued theme in the future of this project.
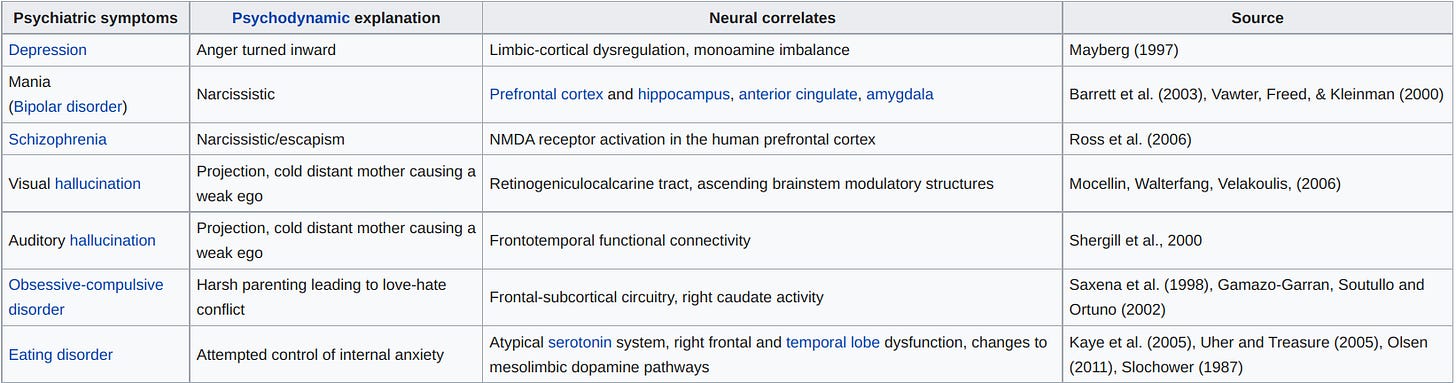
I sense pushback against these categories may be warranted; it is from Wikipedia, after all. I would be interested if readers can point me to another version of research findings pointing to neural correlates of mental health.
I made this term up, if there is a more suitable one, let me know!
So what does it mean when companies are combining digital health products with Psychedelics?