Software Assisted Ketamine Therapy; DEA Pushes Back on Right-to-Try Case;
And other related Ketamine news
A running theme of this newsletter has been the role of technology in psychedelic therapy.
For reasons related to the time and cost associated with therapy, the neuroplastic nature of psychedelics, and the advent of Software As a Medical Device (SAMD, it is almost self-evident that technology-based aftercare will play a significant role in mainstream healthcare settings.
But research in this area is in its infancy.
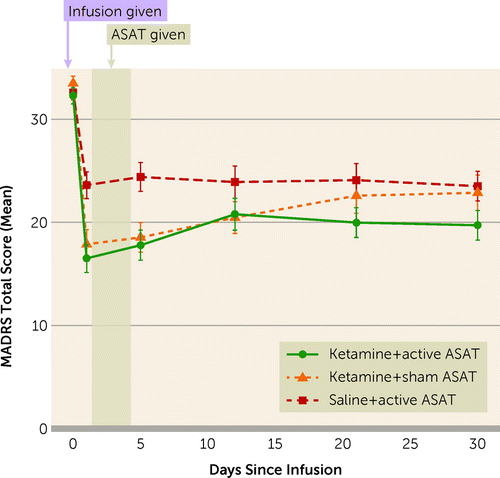
A recent paper—A Novel, Brief, Fully Automated Intervention to Extend the Antidepressant Effect of a Single Ketamine Infusion: A Randomized Clinical Trial—from Rebecca Price at the University of Pittsburgh lends support to the idea that engaging with specific digital technologies after treatment may enhance or extend the therapeutic benefits.
The basic premise of the trial is in the first line of the abstract (emphasis added):
“Intravenous ketamine, which displays rapid antidepressant properties, is posited to reverse depression by rapidly enhancing neuroplasticity. The authors tested whether an automated, computer-based approach could efficiently leverage enhanced neuroplasticity to extend the durability of rapid clinical response.”
In the three-armed study, a single intravenous injection of ketamine combined with computer-based neurocognitive training was compared against ketamine alone and neurocognitive training alone.
Notably, all three groups displayed a reduction in depression, but the combination of ketamine plus neurocognitive training (“Ketamine+ active ASAT”) proved most effective in the 30-day follow-up period.
The neurocognitive training, called Automated Self-Association Training (ASAT), “was designed to leverage Pavlovian conditioning in order to promote positive implicit self-associations and self-worth.”
The program is shockingly simple in that it merely showed study subjects “pictures of smiling faces” and positive language for 40 minutes a day for four days after the ketamine injection.
Lead author Rebecca Price described it in this way:
“…we showed them repeated pairings of self-related words and images—such as the letter “I” and photos of the patient—paired up repeatedly with positive cues. These include positive words like “good,” “sweet,” and “lovable” as well as photos of strangers smiling.”
And the results are promising. From the conclusion section of the abstract:
“After priming the brain with ketamine, training positive self-associations could provide an efficient, low-cost, portable, noninvasive, and highly dissemination-ready strategy for leveraging and extending ketamine’s rapid antidepressant effects.”
Again, here’s the lead author, Rebecca Price, from the press release (emphasis added):
“Using simple conditioning during the period after ketamine treatment, when the brain is receptive to soaking in new information, allows us to go after key features of depression… Training the brain to link perceptions of yourself with positive ideas during this ketamine-primed plasticity window exceeded my expectations. I was surprised and amazed to get such clear findings from an intervention that was so minimal.”
A First
This trial is novel because the study design allowed for the untangling of results from the multiple variables.
“To our knowledge, this represents the first study to test a biobehavioral pairing of ketamine with a behavioral intervention that has included both a no-ketamine and a no-behavioral-intervention control condition. Likewise, clinical trials testing a wide range of posited synergistic treatments (e.g., behavioral treatments paired with “psychoplastogens” or other brain-based targeted approaches, e.g., neuromodulation) have routinely neglected one or the other of these two critical comparators.”
This is exciting territory, and I wish I had a better grasp on the body of research “testing a wide range of posited synergistic treatments” (perhaps a worthwhile research project…).
It is exciting because psychedelic research is limited by the inability to blind study subjects to the subjective effects of the drugs (For a more thorough dive into this, see Great Expectations).
And because we can’t blind psychedelic experiences and easily compare them to inactive placebo, the most promising lines of research are not necessarily “does X psychedelic “work” for Y condition?” but rather, what combination of psychedelic, setting, and support (therapy, Virtual Reality, Journaling, Biofeedback, etc.) are the safest and most effective?
The authors of Great Expectations: Recommendations for improving the methodological rigor of psychedelic clinical trials called this approach Treatment Optimization Research and suggested it could prove valuable:
“…rather than eliminating treatment-nonspecific effects, should trialists be looking for ways to optimize and synergize them with treatment interventions to enhance clinical outcomes?
That is precisely the approach Price, and her colleagues took in this Ketamine+ASAT study and why I thought it was worth looking into.
Ketamine in the Wild: Costs, Safety & Effectiveness
Two related studies on real-world ketamine came out this week.
A comparison of the cost-effectiveness of Esketamine (Spravato) and ketamine by the team at Novamind/Numinus found that:
“esketamine unlikely to be cost-effective under a healthcare sector perspective. Under a patient perspective, esketamine had similar effectiveness and was less costly than ketamine due to insurance coverage.”
In other words, Spravato is affordable for patients with insurance, but it is not cost-effective from the healthcare systems’ perspective because of the higher-than-value cost.
On the other hand, generic ketamine is dirt cheap, but because it is used off-label, it is not covered by insurance, and it is not uncommon to see treatments in the $400-$500 range that patients are responsible for themselves.
Another study, funded by Nue Life, used patient-reported outcomes from electronic medical records to assess the effectiveness and safety of self-administered sublingual ketamine lozenges for depression.
“This study has shown that in as few as three doses of ketamine therapy nearly 50% of patients with moderate to severe depression saw an improvement of reducing their PHQ-9 and GAD-7 scores to half of their intake scores. This reduction rate improved to 60% in patients who completed a clinically recommended six ketamine RDT course of treatment.”
These results echo those found in a similar study from Mindbloom published in July.
Further Reading
Marijuana Moment: DEA Suggests Doctor Seeking Psilocybin For Terminally Ill Patients Has Financial Interest, So It Won’t Waive Records Fee
“The Drug Enforcement Administration (DEA) is suggesting that a doctor seeking psilocybin for terminally ill patients under federal “Right to Try” (RTT) laws has a financial interest that renders him ineligible to get a fee waiver for records requested by his attorney…
Aggarwal and Tucker say that they’ve run up against DEA delay tactics before. But this is the first time that the agency has classified one of their requests as commercial.
As they pointed out to DEA in a letter appealing the designation, RTT law specifically “prohibits the compensation of a physician providing care to a patient for the purpose of accessing an investigational drug.”

Nature: Your brain on psychedelics
“Resting-state fMRI studies have often come to contradictory conclusions, making it difficult to know which theory best explains the effects of psychedelics…It also highlighted many methodological differences, including the drug dose used, how scanning data were processed, and what methods of data analysis were used.”
Marijuana Moment: Colorado Polls Show Conflicting Results On Psychedelics Ballot Measure That Voters Will Decide On In November
“Psychedelics reform is on the ballot in Colorado this November, and two recent polls paint conflicting pictures about how voters will come down on the historic initiative…
It would be a first-of-its-kind law in the U.S. if approved by voters. But the limited polling that’s available has produced divergent results, with one survey finding strong support for the measure (70 percent) and another showing a plurality of voters (41 percent) opposed to the reform.”
Nature: Psychedelic research and the real world
“However, continuing to exclude groups that are highly likely to seek treatment with psychedelics, should they be approved for clinical use, will leave physicians flying blind when these people turn up in the clinic. Once regulatory authorities approve the clinical use of psychedelic medicines, the incentive for pharmaceutical companies to sponsor trials with groups that are perceived as higher risk will plummet.”
The Conversation: Psychedelic drugs: how to tell good research from bad
“Research with psychedelic drugs has made a dramatic comeback amid a heady mix of softening societal attitudes, the lure of commercial opportunity, misgivings about the “war on drugs”, and the desire to develop new ways to treat mental health conditions… In this fervour, which research is worth your time and, more importantly, your trust? Of course, what’s worth your time depends on what you want.”